
Our biostatistics and statistical programming proficiency goes hand-in-hand with our deep knowledge of complex indications and therapeutic areas. Our team successfully predicts risks, prevents delays, saves additional expenses, allocates resources wisely, and creates realistic timeframes thanks to years of experience planning and performing Phase I–IV clinical trials, integrated analytics, patient registries, and regulatory filings.
Our biostatistics and programming team has worked in a range of partnership models, such as full-service work with a project management team, standalone biostatistics and/or programming assistance with other vendors, consultancy support, and specialised resourcing models. We can serve as your primary statistical team to plan and conduct your clinical trial, or we can supplement and oversee your existing statistical team as needed.
Our biostatistics team can provide you with in-depth help for any difficult statistical needs. They join your core team and assist you in ensuring effective research design and planning. They are there to assist you in any manner when faced with difficult situations and decisions.
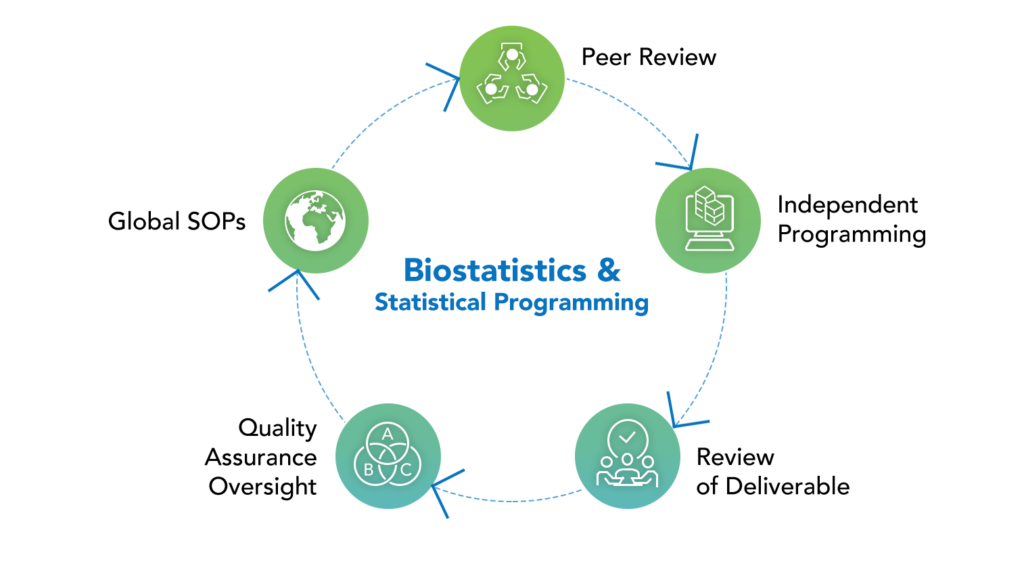
All biostatistics and programming outputs are adaptable and meeting to your specific requirements. Our adaptable approach to statistics and programming services is tailored to small and medium-sized businesses. We have an experienced staff, strong management oversight and participation, a track record of successful regulatory filings, and a broad range of therapeutic experience throughout all research phases.

Submit your details and we’ll be in touch shortly. You can also email us if you would prefer.
Copyrights © 2012 – 2023 ICBIO Corporation